How do glow sticks work?
Before we can begin to understand how glow sticks (chemiluminescence) work, we need to understand their origin. Glow sticks were developed in the 1960’s by a chemist named Edwin Chandross working under government contract for Bell Labs. He developed glowsticks to explain the process of chemicals emitting intense light without giving off heat (chemiluminescence).
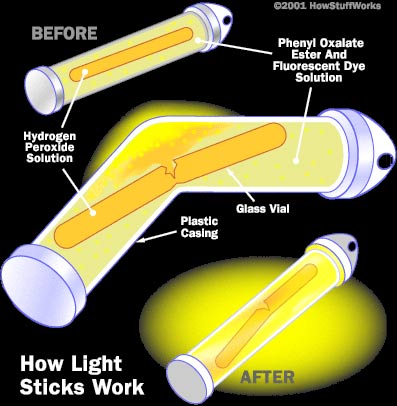
Glowsticks are made up of three main chemicals:
· Hydrogen peroxide
· Phenyl oxalate ester
· Fluorescent dye (Fluorophores)
The Hydrogen peroxide is generally separated by being incased in glass from the Phenyl oxalate ester and fluorescent dye. No reaction will take place so long as these two chemicals remain separated. Glass however is not always used to separate the Hydrogen peroxide.
Have you ever heard of dry ice glow sticks?
Dry ice glow sticks are manufactured in a glowing state with all three chemicals already combined and put on dry ice to stop the chemiluminescence process from occurring. Once the glow sticks are removed from the dry ice and allowed to warm, the chemiluminescence process will take hold and they start to glow.
.png)
Now that we know the basic composition of a glow stick, where does the color come from?
Here are a list of other fluorophores used in the fluorescent dyes:
· orange light is emited from 5,12-bis(phenylethynyl)naphthacene
· green light is emited from 2-chloro-9,10-bis(phenylethynyl)anthracene
· yellow light is emited from 1,8-dichloro-9,10-bis(phenylethynyl)anthracene
